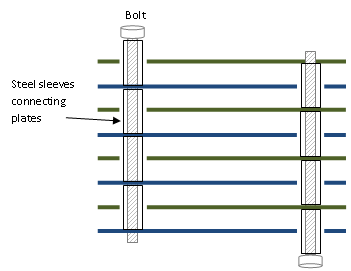
Our first try of the oxyhydrogen generator was a stack of stainless steel plates with bolts through. The problem was there was pretty poor contact between the bolts and the plates so I've changed it to use metal sleeves between the plates as below.
|
So I found a little stainless steel tube in a box of old copier parts that neatly fitted over the stainless bolts I had to bolt the plates together.
I cut that into 10mm lengths (twice the plate spacing) and covered with heatshrink. I just stuck all 16 spacers into a length of heatshrink, heated it then cut them and trimmed them up so the steel was clear at each end. I also cut up some plastic tubes that were big enough to fit a steel spacer with heatshrink on inside it (I used some old pens). They were about 4.5mm long (4.5+4.5+thickness of steel=10mm). 32 of them. You can probably do without those if your steel is a bit thicker but the steel I used was really thin so they just help keep it spaced right.

|
Then it was just matter of bolting them all together, staggering the spacers to make alternating plates connected to each other. I just stacked all the sheets together and drilled four snug bolt holes, putting a bolt in each as I drilled to keep everything lined up (I numbered the plates to get the order right later on). I made the two opposite corners share the same pole. So you can see the next plate that goes on will touch the steel tube on the lower right and upper left, but the other two go through that plate and touch the next plate. I fairly generously enlarged the holes of the corners where a spacer goes through a plate to give a reasonable clearance between the plate and the heatshrink covered steel spacer - but not wider than the plastic spacer :)

|
You can see the staggering below. You need to put spacers on the ends of the appropriate bolts to keep them away from the wrong plate. Then I just bolted it up tight to get the steel spacers pushing good and hard into each plate.
You can see that when I cut the steel plates I left two plates with long tabs to connect to the power source. I used the 3rd and 6th plate as the connectors because it fitted the jar lid well, but I don't imagine it would matter.

|
And running very briefly sucking 15A! It's running too hot for the wires at that current but I had 2 tsp of baking soda in the ~900ml jar.

|
We filled a balloon with oxyhydrogen and set it off with a long string. It might not look it but it's a big bang. You would not want be anywhere near this and it could be set off pretty easily. Even small bubbles of oxyhydrogen give a big bang. It's probably best to use it as you go and not store any quantity of it.
|